There is a close relationship between dissolved oxygen and biochemical oxygen demand. BOD is the amount of dissolved oxygen required to break down the organic material of the sample water by aerobic biological organisms at certain temperature over a specific time period. Generally this parameter is determined with the consumption of oxygen by the micro organism during 5 days of incubation at 20⁰C. It is expressed as milligrams/liter or ppm. It is also known as biological oxygen demand. You should remember that BOD is an indicator and not a pollutant.

Importance of BOD
BOD is the best and reliable method to determine the level of pollution by the organic waste. Although it has some natural limitation but it is a significant method. It is important method to analysis the sewerage, industrial, effluent and extensive pollutant water. If one liter of sample water consumes 100mg of oxygen to biodegrade of organic matter, then the BOD is 100 ppm. The BOD value of drinking water should have less than 1 ppm. On the other hand the BOD value of raw sewerage runs from 200 ppm to several hundred ppm.
BOD indicates the amount of pollution of water bodies. Lower BOD indicates the water is good quality for aquatic life or little aerobic activity whereas higher BOD indicates the water is highly polluted. When the BOD is higher, then DO becomes lower. All the aquatic animals rely on this dissolved oxygen to live. So the reduction of DO in the water can bring a negative effect on the fish and other aquatic life. When it is drops below a certain level, the aquatic life are unable to continue at a normal rate. Aquatic organisms become stressed, asphyxiate, and may die.
Sources of organic materials
Common sources of organic materials are plant decay, leaves, grass clippings, woody debris; animal wastes; wastewater from residential areas, food processing plants, dairy plants, pulp and paper mills, canneries; septic systems leakage; fertilizer runoff and urban storm-water runoff. These organic materials run into water bodies and increases oxygen demand. Dead algae or other organisms are also part of the decomposition cycle. They are responsible for water pollution because they stimulate the growth of micro organisms that can increase the biochemical oxygen demand. All most all natural water contain small amount of organic materials.
Determination of BOD
The most common and popular method for determination of Biochemical Oxygen Demand is Standard Method which is recognized by U.S. EPA and leveled as 5210B. It is not a accurate quantitative test, although it is widely used as an indication of water pollution.
BOD test procedure
- At first analyzed and conditioned the water sample to ensure favorable growth conditions for bacteria, which may include adjustment for pH (6.5-7.5), neutralization of residual chlorine, or reduction of DO in supersaturated samples. If there is no or less oxygen in any sample then oxygen is provided to the sample water. To provide oxygen entered air into the water sample with fusion tube up to 5 minutes or the DO level up to 7 ppm.
- If BOD is higher than DO, then dilute the sample water with BOD free water (distilled water) to lower the BOD level.
- Then added the appropriate amount of seed bacteria. The selection of micro organisms (seed bacteria) is very important and the results are obviously not reproducible.
- Determination the initial DO (D1) of one portion of the sample.
- Then rest of the dilute sample filled into a 250-300ml incubation bottle. The sample incubates for 5 days in the dark room at 20 °C to prevent DO production via photosynthesis. You can cover the sample bottle completely with aluminum foil.
- After the 5 days, the sample is removed from the incubator and the bottles uncork, then take the final dissolved oxygen (D2) reading.
- The difference between the first and the last of the samples is called the BOD.
BOD calculation
Calculate the BOD from the DO depletion and volume of sample used following the formula below:
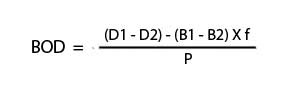
Where:
D1 is the DO of the sample after dilution (ppm).
D2 is the DO of the diluted sample after 5 day incubation (ppm).
B1 is the DO of diluted seed sample after preparation (ppm).
B2 is the DO of diluted seed sample after 5 day incubation (ppm).
f is the ratio of seed volume in dilution solution to seed volume in BOD test on seed.
P is the decimal dilution factor. [For example, if a sample of 100 ml dilute into 500 ml, then P = 100/500 = 0.20].
BOD of some collected sample from different sources:
| Sample | BOD | Remarks |
|---|---|---|
| Normal water | 0-3 | Acceptable |
| River water | 5-20 | Polluted |
| Sewerage water | 50-100 | Very bad water |
| Industrial water | 150-1000 | Worst water |