Arsenic is a poisonous element that is found in water and soil. It can cause serious health problems, so it’s important to test your water for arsenic levels. In this blog post, we will discuss how to choose the right arsenic water test kit for your needs. We’ll also provide some tips on how to use the test kit correctly. So if you’re concerned about the safety of your drinking water, read on!
What is arsenic and why do we need to test for it in our water supply?
Arsenic is a chemical element that can be found in water, air, and soil. It’s odorless and colorless, so you can’t see or smell it. Arsenic is poisonous and exposure to high levels can cause serious health problems. That’s why it’s important to test your water for arsenic levels.
There are two types of arsenic: inorganic and organic. Inorganic arsenic is the more dangerous type, but both types can be harmful to your health. The EPA has set a maximum contaminant level (MCL) for arsenic in drinking water at 0.010 mg/L. This means that water with an arsenic concentration above this level is not safe to drink.
If you’re on a private well, it’s your responsibility to test your water for arsenic and other contaminants. You should test your water at least once a year. If you live in an area where the water is known to be contaminated with arsenic, you may need to test more often.
How does an arsenic water test kit work and what do the results mean for your health and safety?
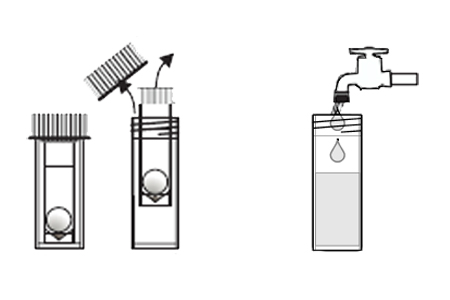
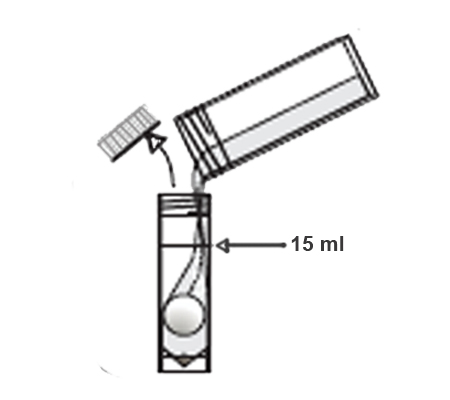
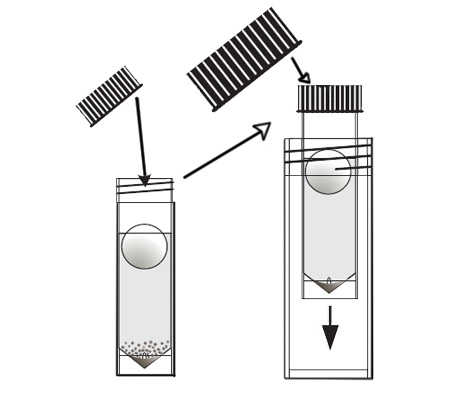
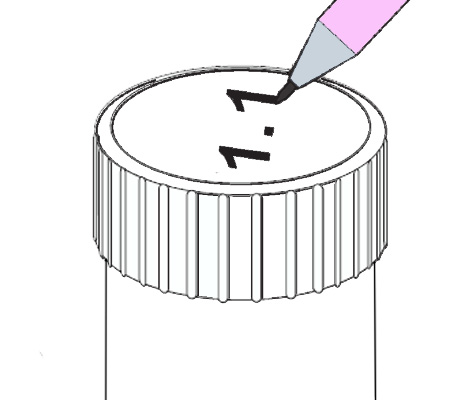
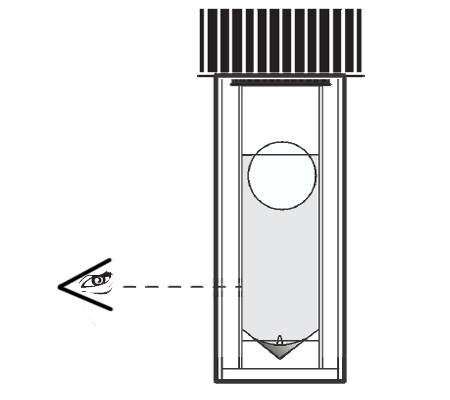
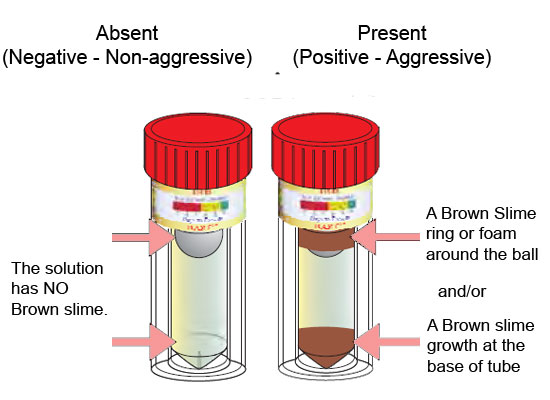
An arsenic water test kit usually comes with a vial of liquid reagent and a color chart. To use the kit, you’ll need to collect a sample of your water in a clean container. Then, you’ll add a few drops of the reagent to the water and compare the color of the water to the chart.
The color chart will tell you the arsenic concentration in your water. If the arsenic level is above the EPA’s MCL, your water is not safe to drink and you’ll need to take steps to remove the arsenic from your water supply.
What are some of the best arsenic water test kits on the market today, and how much do they cost?
There are many different types of arsenic water test kits available on the market. Some are designed for home use, while others are meant for commercial or industrial applications. The type of kit you need will depend on the amount of water you want to test, as well as your budget. For example, if you only need to test a small amount of water, a home kit may be sufficient. However, if you’re testing large quantities of water, you’ll need a more sophisticated and expensive commercial-grade kit.
Here are a few of the best arsenic water test kits on the market:
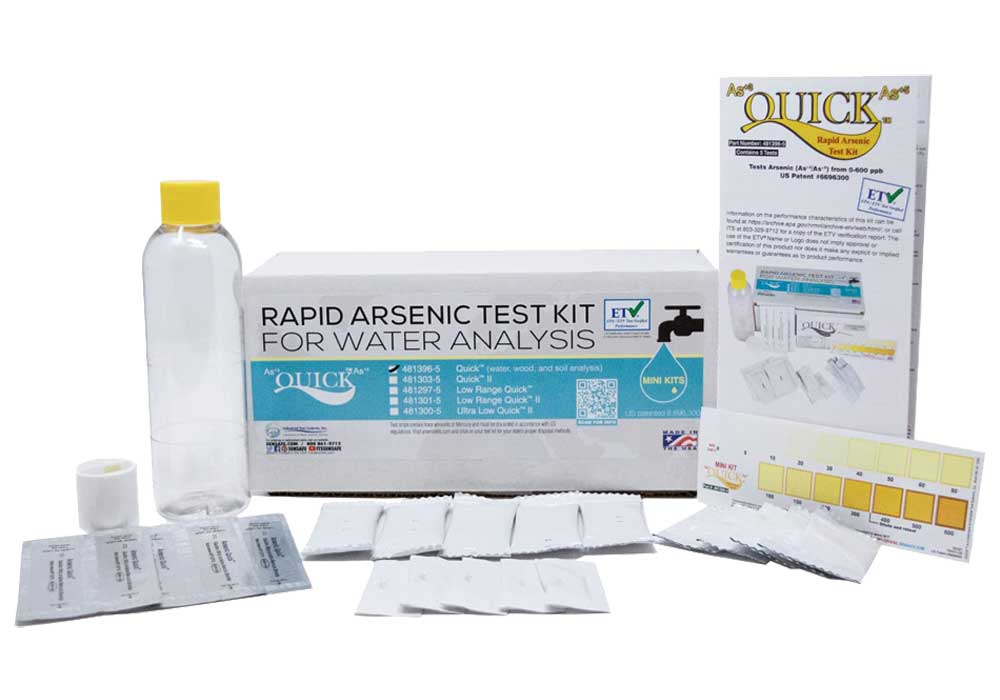
– Industrial Test Systems Quick 481396-5 Arsenic water Test Kit is one of the most popular options and it’s very affordable, costing a bit more expensive. This kit can measure levels as low as 0.005 mg/L, which is below the EPA’s MCL.
– The Hach Arsenic Test Kit is another popular choice and it’s a bit more expensive, costing around $30. This kit can measure levels as low as 0.001 mg/L, which is well below the EPA’s MCL.
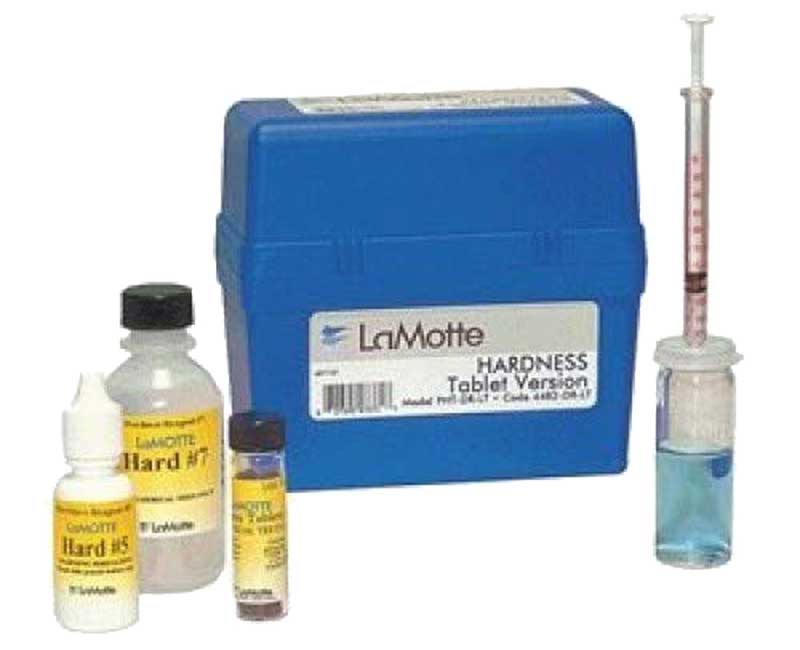
– The LaMotte Arsenic Test Kit is a more expensive option, costing around $60. This kit can measure levels as low as 0.0001 mg/L, which is incredibly sensitive and can give you a very accurate picture of your water’s arsenic levels.
Choosing arsenic water test kit
When choosing an arsenic water test kit, it’s important to make sure that it can measure levels below the EPA’s MCL. It’s also important to read the instructions carefully. This will ensure that you’re using the kit correctly and getting accurate results. Pay attention to the recommended sampling methods and follow them closely. If you’re unsure about anything, don’t hesitate to contact the manufacturer for clarification. Remember, you can’t see or smell arsenic, so the only way to know if it’s in your water is to test for it.
Make sure you understand all of the steps involved in the testing process. If possible, practice using the kit on a small quantity of water before testing your drinking supply. This will help you get a feel for how the kit works and ensure that you’re using it correctly.
How often should you test your water for arsenic, and what should you do if levels are high or unsafe?”
You should test your water for arsenic at least once a year. If you live in an area where the water is known to be contaminated with arsenic, you may need to test more often.
If your water has high levels of arsenic, you’ll need to take steps to remove it from your water supply to protect your health. Arsenic exposure can cause a variety of health problems, including cancer. So, don’t delay in taking steps to remove it from your water supply. There are a few different ways to remove arsenic from water. One option is to install an arsenic removal system. These systems are usually expensive, but they’re very effective at removing arsenic from water.
Another option is to use bottled water for drinking and cooking. This is a more affordable option, but it’s not always convenient.
Conclusion
Now that you know more about arsenic water test kits, you can make an informed decision about which one is right for you. Remember, it’s important to test your water regularly for arsenic and take action if levels are high. By taking these simple steps, you can protect your health and ensure that your family has safe, clean water to drink.